Imagine Victorian London, but its skies are filled with airplanes. Steam robots crowd the streets, mingling with people in hats and cloaks. This kind of retrofuturistic mash-up is the fantasy realm of steampunk, a genre of literature, film, and other creative media. Theoretical physicist Nicole Yunger Halpern sees her specialty, the field of quantum thermodynamics, as the real-life version of steampunk.
In steampunk, “there’s a strange juxtaposition of old environment and futuristic technology,” says Yunger Halpern. “That’s what we do in quantum thermodynamics.”
Thermodynamics, developed in the 1800s in the context of the Industrial Revolution, describes the physics concepts of heat, work, and energy (SN: 6/12/24). The field arose out of scientific efforts to understand steam engines. Unlike the clatter and clatter of industrial machines, quantum physics describes phenomena at the scale of atoms, electrons, and the like, and has fueled the development of modern technologies such as quantum computers (SN: 28/6/23).
In the past, some physicists didn’t think the idea of quantum thermodynamics made sense. “They saw it as an oxymoron,” says Yunger Halpern.
Now, however, the two concepts collide in quantum engines and other miniature devices. Quantum thermodynamics researchers aim to develop the tools to describe heat, work, cooling, and efficiency in quantum systems and to determine the performance limits of quantum devices. Yunger Halpern, a National Institute of Standards and Technology physicist based at the Joint Center for Quantum Information and Computer Science in College Park, Md., is at the forefront of these efforts.
“She has a vision and follows it,” says quantum physicist Aram Harrow of MIT. “She’s also good at recruiting other people to her vision.”
One of Yunger Halpern’s major contributions has been exploring what the quantum concept behind Heisenberg’s uncertainty principle might mean for thermodynamics.
Imagine a hot cup of tea. Thermodynamics describes how energy moves from the tea to the surrounding air, or how evaporating water molecules escape. Both of these quantities—energy and water molecules—are conserved in this scenario, meaning they can move from one place to another, but the total amount is fixed. The problem of explaining how conserved quantities are exchanged occurs repeatedly in thermodynamics.
Now, what if the tea wasn’t a whole cup, but a packet of just a few atoms? Yunger Halpern wants to know how the exchange would change. In quantum physics, conserved quantities can be mutually exclusive. This means that they cannot be measured simultaneously. Heisenberg’s Uncertainty Principle, which states that the better you know the position of a quantum object, the worse you know its momentum and vice versa., give a famous example (SN: 1/12/22).
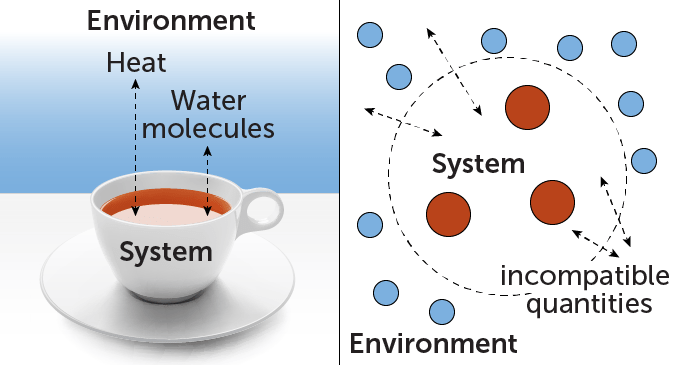
“For many decades, almost nobody thought about what happens when you have a system and environment that exchange quantities that are incompatible,” says Yunger Halpern. It turns out that incompatibility can have a real impact on how the system behaves, she and colleagues noted in a survey of the topic published in 2023 in Nature Reviews Physics. For example, incompatibility can reduce the amount of entropy, or disorder, that is produced in such exchanges. Because the total entropy of an isolated system tends to increase with time, some scientists think that entropy is closely related to an “arrow of time” that distinguishes the future from the past.SN: 7/10/15). In a sense, says Yunger Halpern, this means that incompatible quantities can hinder a system’s ability to experience that arrow of time.
Quantum thermodynamics has led to some neat laboratory demonstrations. For example, a single atom can be turned into a quantum engine that converts heat into work (SN: 14.4.16). Now, Yunger Halpern aims to put quantum thermodynamics to practical use through autonomous quantum machines.
Typical quantum devices, such as single-atom engines, atomic clocks, or the quantum parts that make up quantum computers, require constant prodding by experimenters to operate. Autonomous devices will operate automatically.
Yunger Halpern joined colleagues to bring this idea to reality. The result was an autonomous quantum refrigerator that can automatically cool a quantum bit, the team reported in May 2023 on arXiv.org.
And in a July 2023 arXiv article, she and colleagues laid out the criteria that must be met to create an autonomous quantum machine. For example, these machines must have structural integrity and sufficiently pure quantum states. In addition, their output must be worth the input required to execute them. This means that a quantum motor cannot take in more energy to control it than it puts out. Quantum physicist Marcus Huber worked with Yunger Halpern in developing these criteria. “I found it brilliant, but also mega intense and focused,” says Huber, from TU Wien in Vienna. “She will bombard you with relevant and good questions.”
It’s not just her science that’s in the spotlight—her writing is, too. Yunger Halpern’s book 2022, Quantum Steampunk: Yesterday’s Tomorrow’s Physicsattracted the attention of the public in the field. She is also a science blogger at the website Quantum Frontiers. Writing, Yunger Halpern says, allows him to explore new ideas without the constraints of scientific publishing (fantasy speculations and “out there” ideas aren’t likely to pass peer review). “Thinking really big and wild and so creatively that you feel like thinking on a certain day of the month is helpful for keeping creativity in physics.”
And just as her work juxtaposes old and new, Yunger Halpern often illustrates contrasts, says Shayan Majidy of the University of Waterloo in Canada and soon to join Harvard University, who recently completed his Ph.D. advised by Yunger Halpern. She holds her students to high standards, but is warm and caring as a counselor. Majidy says that when he got married, Yunger Halpern somehow figured out his favorite local ice cream brand — Kawartha Dairy — and sent him a gift card.
Her hobbies tend towards quiet and slow-paced activities: walks, museum visits. Yet she injects intense passion into her work. “She has very old-fashioned interests and tastes,” Majidy says, “but she’s this very young, energetic researcher of rising stars.”
#physicist #bringing #thermodynamics #quantum #age
Image Source : www.sciencenews.org